Amidation of triglycerides by amino alcohols and their impact on plant oil-derived polymers†
Abstract
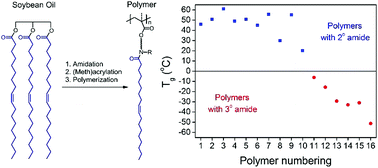
Amidation of plant oils with amino alcohols was methodologically examined. Twenty one amino alcohols, varying in alcohol substitutions, linkers and amino substitutions, were respectively reacted with high oleic soybean oil. The structural factors of amino alcohols controlled their reactivity in amidation. While most of them resulted in quantitative conversion of triglycerides, steric hindrance on secondary amines resulted in much lower yields. Subsequent synthesis and radical polymerization of (meth)acrylates led to polymers with a distinct dependence essentially originating from the amino alcohols. Depending on the backbone and amide structures in the side chain, these polymers exhibited wide glass transition temperatures with a difference of more than 100 °C, ranging from viscoelastic materials to thermoplastics. A proof-of-concept hydrogenation of unsaturated double bonds was carried out, providing an approach to precisely controlling the thermal and mechanical properties of plant oil-derived polymers.

 Please wait while we load your content...
Please wait while we load your content...