Chemo- and regioselective reactions of 5-bromo enones/enaminones with pyrazoles†
Abstract
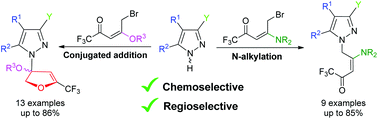
Reaction of 5-bromo enones with pyrazoles provided a series of unexpected N,O-aminal derivatives, through a 1,4-conjugated addition at the β-carbon of the 5-bromo enones instead of the expected nucleophilic substitution of the bromine. This reaction also furnished the 1,3-regioisomer of the pyrazole. A similar reaction of pyrazoles using 5-bromo enaminones furnished only N-alkylated pyrazoles—with high regioselectivity and at good yields—through nucleophilic substitution of the bromine.
- This article is part of the themed collections: Celebrating Latin American Talent in Chemistry and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...