The solvent-controlled chemoselective construction of C–S/S–S bonds via the Michael reaction/thiol coupling of quinoline-2-thiones†
Abstract
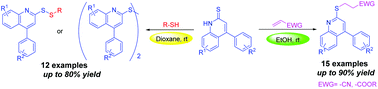
K2CO3-catalyzed thio-Michael addition using quinoline-2-thiones and α,β-unsaturated carbonyl compounds was used to assess the chemoselective construction of C–S and S–S bonds under mild reaction conditions in different solvents. The C–S bond showed a better chemoselective construction in EtOH whereas the S–S bond showed a better chemoselective construction in 1,4-dioxane. The corresponding products, generated from the reaction, presented a significant solvent-controlling effect.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...