Synthesis of resolvin E3 via palladium-catalyzed addition of AcOH to vinyl epoxy alcohols†
Abstract
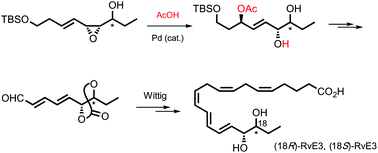
(18R)- and (18S)-stereoisomers of resolvin E3 (RvE3), potent anti-inflammatory mediators, were synthesized stereo- and enantioselectively through the Wittig reaction of the carbonate of 6R,7R- and 6R,7S-dihydroxynona-2E,4E-dienal, a C12–C20 part, with the phosphonium salt corresponding to the C1–C11 part. The stereoisomeric carbonate was prepared by the Swern oxidation of 3-(AcO)-6R,7R- or 3-(AcO)-6R,7S-(dihydroxy-carbonate)-4-nonen-1-ol followed by the spontaneous elimination of the AcO group in one pot. The (6R,7R)-(dihydroxy-carbonate)-alcohol for (18R)-RvE3 was, in turn, provided by stereoselective epoxidation of 9-(TBS-oxy)nona-4Z,6E-dien-3R-ol with m-CPBA and the subsequent Pd-catalyzed addition of AcOH to the resulting syn vinyl epoxy alcohol followed by carbonate formation of the vic-syn-diol and TBS desilylation. The Mitsunobu inversion of the syn vinyl epoxy alcohol gave the anti isomer, which was converted to 3-(AcO)-6R,7S-(dihydroxy-carbonate)-4-nonen-1-ol, the intermediate to (18S)-RvE3, by the same set of reactions.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...