Asymmetric total synthesis of (+)-O-methylasparvenone, a rare nitrogen-free serotonin 2C receptor antagonist†
Abstract
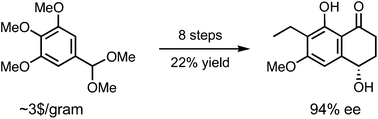
The first enantioselective synthesis of the fungal metabolite (+)-O-methylasparvenone was achieved in eight steps and 22% overall yield from inexpensive 3,4,5-trimethoxybenzaldehyde dimethyl acetal. Key steps include (i) early-stage asymmetric alkynylation of an aromatic aldehyde with a propiolate, (ii) intramolecular Friedel–Crafts acylation, and (iii) site-selective cleavage of an aryl methyl ether.

 Please wait while we load your content...
Please wait while we load your content...