Bifunctional aryloxyphosphoramidate prodrugs of 2′-C-Me-uridine: synthesis and anti-HCV activity†
Abstract
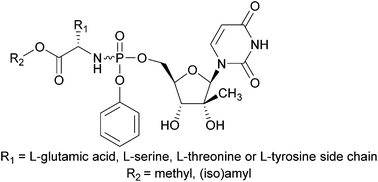
In an attempt to identify novel nucleoside phosphoramidate analogues for improving the anti-HCV activity of 2′-C-Me-uridine, we have synthesized for the first time a series of L-glutamic acid, L-serine, L-threonine and L-tyrosine containing aryloxyphosphoramidate prodrugs of 2′-C-Me-uridine. Evaluation of their activity against HCV revealed that they displayed very potent anti-HCV activity, with EC50 values that are in the same range as of Sofosbuvir.

 Please wait while we load your content...
Please wait while we load your content...