Racemic and enantioselective metal-catalyzed synthesis of SF5-containing diarylmethanols†
Abstract
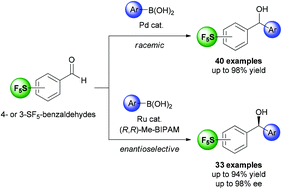
The racemic and enantioselective metal-catalyzed addition of arylboronic acids to 4- and 3-(pentafluorosulfur)benzaldehydes is reported. The racemic synthesis was accomplished using a Pd-based system and a wide range of arylboronic acids could be used, resulting in yields of 42 to 98% of the corresponding SF5-containing diarylmethanols. A ruthenium-based system, along with (R,R)-Me-BIPAM as the chiral ligand, was investigated and optimized for the enantioselective version. In this case, while the chiral SF5-containing diarylmethanols were generally obtained in good yields (up to 94%) and enantioselectivities (up to 98% ee), limitations were also observed. For instance, 4-(pentafluorosulfur)benzaldehyde generally provided slightly better yields than 3-(pentafluorosulfur)benzaldehyde. In addition, lower yields and enantioselectivities were observed when using either 4- and 3-substituted arylboronic acids bearing electron-withdrawing (e.g., CO2Et, NO2, CF3) or 2-substituted arylboronic acids (regardless of the nature of the substituent). Overall, the SF5-containing diarylmethanols described herein represent novel and potentially useful fluorinated building blocks for the synthesis of biologically active compounds.

 Please wait while we load your content...
Please wait while we load your content...