Computational insight into the cooperative role of non-covalent interactions in the aza-Henry reaction catalyzed by quinine derivatives: mechanism and enantioselectivity†
Abstract
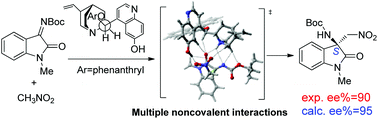
Density functional theory (DFT) calculations were performed to elucidate the mechanism and the origin of the high enantioselectivity of the aza-Henry reaction of isatin-derived N-Boc ketimine catalyzed by a quinine-derived catalyst (QN). The C–C bond formation step is found to be both the rate-determining and the stereo-controlled step. The results revealed the important role of the phenolic OH group in pre-organizing the complex of nitromethane and QN and stabilizing the in situ-generated nitronate and protonated QN. Three possible activation modes for C–C bond formation involving different coordination patterns of catalyst and substrates were studied, and it was found that both the ion pair-hydrogen bonding mode and the Brønsted acid-hydrogen bonding mode are viable, with the latter slightly preferred for the real catalytic system. The calculated enantiomeric excess (ee) favouring the S enantiomer is in good agreement with the experimental result. The high reactivity and enantioselectivity can be ascribed to the cooperative role of the multiple non-covalent interactions, including classical and non-classical H bonding as well as anion⋯π interactions. These results also highlight the importance of the inclusion of dispersion correction for achieving a reasonable agreement between theory and experiment for the current reaction.

 Please wait while we load your content...
Please wait while we load your content...