Traceless reductive ligation at a tryptophan site: a facile access to β-hydroxytryptophan appended peptides†
Abstract
An efficient methodology for cysteine-free ligation at a tryptophan (Trp) site is described. A chemically active scaffold, β-hydroxy-α-azidotryptophan, has been synthesized and explored towards the synthesis of a series of β-hydroxytryptophan appended native peptides in good yields via one-pot reductive traceless ligation of β-O-peptidyl-α-azidotryptophan involving an O → N peptidyl transfer strategy. Pre-organized conformational analysis and reaction energy pathway based theoretical studies further supported the experimental findings on the chemical structure stability of ligated products.
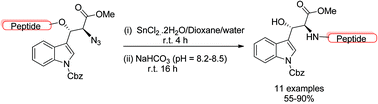

 Please wait while we load your content...
Please wait while we load your content...