Synthesis and biological evaluation of triazole based uracil derivatives as novel DPP-4 inhibitors†
Abstract
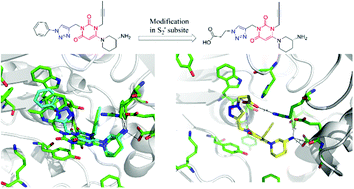
A series of triazole based uracil derivatives were designed and synthesized as novel DPP-4 inhibitors. Compound A01 was identified as a lead compound for SAR studies focused on the structural modification at the S2′ subsite of DPP-4. The novel analogues A02–A25 were obtained by modifying the substituents at the phenyl group, and B01–B09, by introducing the carbonyl group. On screening in DPP-4, compounds B03, B04 and B08 showed a significant improvement in DPP-4 inhibitory activities compared to compound A01 and showed comparable activities to the marketed DPP-4 inhibitor, alogliptin. Docking studies revealed new favorable binding modes of designed compounds in the S2′ subsite and proved that structural modifications in the S2′ subsite were an effective option to increase the inhibition of DPP-4. In vitro DPP-8 and DPP-9 tests indicated that all compounds showed excellent selectivity against DPP-8 and DPP-9. Further in vivo evaluation showed that compound B04 could significantly improve oral glucose tolerance in ICR mice and dose-dependently reduced glucose levels in type 2 diabetic C57BL/6 mice. These data suggest that compound B04 could be a promising DPP-4 inhibitor for future treatment of T2DM.

 Please wait while we load your content...
Please wait while we load your content...