Investigations of possible prodrug structures for 2-(2-mercaptophenyl)tetrahydropyrimidines: reductive conversion from anti-HIV agents with pyrimidobenzothiazine and isothiazolopyrimidine scaffolds†
Abstract
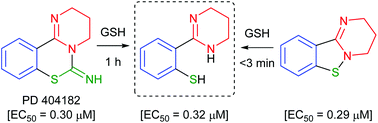
3,4-Dihydro-2H,6H-pyrimido[1,2-c][1,3]benzothiazin-6-imine (PD 404182) and 3,4-dihydro-2H-benzo[4,5]isothiazolo[2,3-a]pyrimidine are the heterocyclic antiretroviral agents against human immunodeficiency virus type 1 (HIV-1) infection. On the basis of similar structure–activity relationships of anti-HIV activities toward the early-stage of viral infection between these unique scaffolds, the transformations under the bioassay conditions were investigated. The distinctive S–N bond in the isothiazolopyrimidine scaffold was immediately cleaved under reductive conditions in the presence of GSH to generate a thiophenol derivative. A similar rapid conversion of PD 404182 into the same thiophenol derivative was observed, suggesting that pyrimidobenzothiazine and isothiazolopyrimidine scaffolds may work as prodrug forms of the common bioactive thiophenol derivatives.

 Please wait while we load your content...
Please wait while we load your content...