Synthesis, antitumor activity, and mechanism of action of 6-acrylic phenethyl ester-2-pyranone derivatives†
Abstract
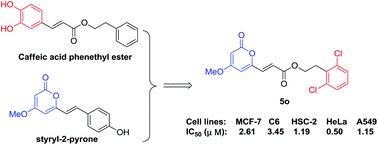
Based on the scaffolds of caffeic acid phenethyl ester (CAPE) as well as bioactive lactone-containing compounds, 6-acrylic phenethyl ester-2-pyranone derivatives were synthesized and evaluated against five tumor cell lines (HeLa, C6, MCF-7, A549, and HSC-2). Most of the new derivatives exhibited moderate to potent cytotoxic activity. Moreover, HeLa cell lines showed higher sensitivity to these compounds. In particular, compound 5o showed potent cytotoxic activity (IC50 = 0.50–3.45 μM) against the five cell lines. Further investigation on the mechanism of action showed that 5o induced apoptosis, arrested the cell cycle at G2/M phases in HeLa cells, and inhibited migration through disruption of the actin cytoskeleton. In addition, ADMET properties were also calculated in silico, and compound 5o showed good ADMET properties with good absorption, low hepatotoxicity, and good solubility, and thus, could easily be bound to carrier proteins, without inhibition of CYP2D6. A structure–activity relationship (SAR) analysis indicated that compounds with ortho-substitution on the benzene ring exhibited obviously increased cytotoxic potency. This study indicated that compound 5o is a promising compound as an antitumor agent.

 Please wait while we load your content...
Please wait while we load your content...