Synthesis, conformational studies, and biological properties of phosphonomethoxyethyl derivatives of nucleobases with a locked conformation via a pyrrolidine ring†
Abstract
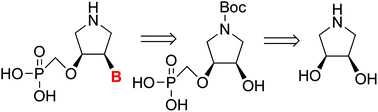
Systematic structure–activity studies on a diverse family of nucleoside phosphonic acids has led to the development of potent antiviral drugs such as HPMPC (CidofovirTM), PMEA (AdefovirTM), and PMPA (TenofovirTM), which are used in the treatment of CMV-induced retinitis, hepatitis B, and HIV, respectively. Here, we present the synthesis of a novel class of acyclic phosphonate nucleotides that have a locked conformation via a pyrrolidine ring. NMR analysis of these compounds revealed that the pyrrolidine ring has a constrained conformation when in the cis-form at pD < 10 via hydrogen bonding. Four of these compounds were tested as inhibitors of the human and Plasmodium falciparum 6-oxopurine phosphoribosyltransferases. The most potent has a Ki of 0.6 μM for Plasmodium falciparum HGXPRT.

 Please wait while we load your content...
Please wait while we load your content...