A biotin-conjugated pyridine-based isatoic anhydride, a selective room temperature RNA-acylating agent for the nucleic acid separation†
Abstract
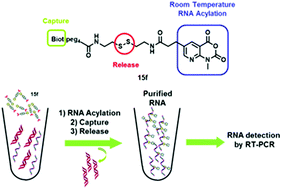
Isatoic anhydride derivatives, including a biotin and a disulfide linker were specifically designed for nucleic acid separation. 2′-OH selective RNA acylation, capture of biotinylated RNA adducts by streptavidin-coated magnetic beads and disulfide chemical cleavage led to isolation of highly enriched RNA samples from an initial 9/1 DNA–RNA mixture. Starting from the parent compound N-methylisatoic anhydride A which was used at 65 °C, we improved the extraction process by designing a new generation of isatoic anhydrides that are able to react under smoother conditions. Among them, a pyridine-based isatoic anhydride derivative 15f was found to be reactive at room temperature, leading to enhance the efficiency and selectivity of the extraction process by significantly reducing DNA side extraction. The extracted and purified RNAs can then be detected by RT-PCR.

 Please wait while we load your content...
Please wait while we load your content...