The cooperative effect of Lewis pairs in the Friedel–Crafts hydroxyalkylation reaction: a simple and effective route for the synthesis of (±)-carbinoxamine†
Abstract
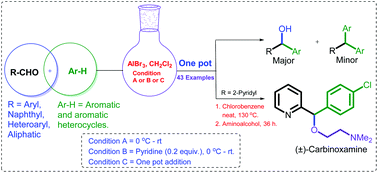
An efficient C–C bond formation strategy between aromatic/heteroaromatic π-nucleophiles and Lewis acid activated aldehydes is described. This aromatic electrophilic substitution reaction of arenes or heteroarenes is facilitated by Lewis acid AlBr3. Aromatic rings with electron donating substituents are excellent nucleophilic counterparts in this reaction, generating carbinols in excellent yields (61–94%). The formation of triarylmethanes is also witnessed in the case of certain reactive aldehydes and aromatic π-nucleophiles through reactive carbocation formation. The formation of triarylmethane is reduced to a greater extent via retardation of the second π-nucleophile addition through a Lewis base, for example, pyridine, coordination with an aluminium alkoxide intermediate. Various aliphatic aldehydes also underwent Friedel–Crafts type hydroxyalkylation and generated the expected carbinols in moderate yields (41–53%) in the presence of AlBr3. This protocol has been successfully applied to the synthesize of the (±)-carbinoxamine, a therapeutically important histamine H1 antagonist, in a one-pot manner.

 Please wait while we load your content...
Please wait while we load your content...