Total synthesis of (+)-phorboxazole A, a potent cytostatic agent from the sponge Phorbas sp.
Abstract
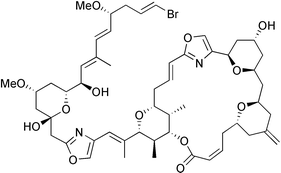
A convergent total synthesis of phorboxazole A (1a), from the C(3–19), C(20–27) and C(33–46) fragments 5, 4 and 91, respectively, concentrating on stereocontrolled formation of the bonds at C(2–3), C(19–20) and C(27–28), is described. Although a coupling reaction between a macrolide ketone and the side chain substituted sulfone, at C(27–28) was not successful, a Wadsworth–Emmons olefination involving the oxane methyl ketone 4 and an oxazole produced the oxane 90 which was next coupled to 91 leading to the C(20–46) unit 100. A further coupling of 100 to 71c at C(19–20) then led to 105, ultimately, and the synthesis was completed by a macrocyclisation reaction from 105, at the C(2–3) alkene bond, followed by deprotection of 106.

 Please wait while we load your content...
Please wait while we load your content...