A remote optically controlled hydrolase model based on supramolecular assembly and disassembly of its enzyme-like active site†
Abstract
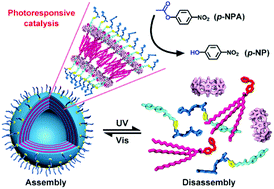
A photoresponsive hydrolase model was constructed through the spatial organization of histidine/arginine-containing peptide supra-amphiphiles that are held together by cucurbit[8]uril (CB[8]) methylviologen (MV) azobenzene (Azo) ternary complexation and subsequently self-assemble into highly uniform giant vesicles. The reversible morphological transition of the vesicular structures to non-assembled peptide fragments was triggered by azobenzene photoisomerization. This enables the assembly/disassembly of its enzyme-like active site to cause a dramatic change in hydrolytic activity. The dynamic process can be directly monitored to determine the supramolecular structure-related enzymatic parameters, which may help to understand how the regulation of enzyme activity is coupled to the aggregation behaviors of natural enzymes.


 Please wait while we load your content...
Please wait while we load your content...