Ion-induced assemblies of highly anisotropic nanoparticles are governed by ion–ion correlation and specific ion effects†
Abstract
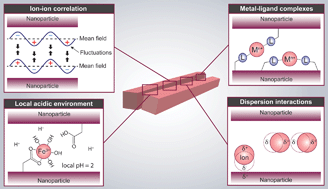
Ion-induced assemblies of highly anisotropic nanoparticles can be explained by a model consisting of ion–ion correlation and specific ion effects: dispersion interactions, metal–ligand complexes, and local acidic environments. Films of cellulose nanofibrils and montmorillonite clay were treated with different ions, and their subsequent equilibrium swelling in water was related to important parameters of the model in order to investigate the relative importance of the mechanisms. Ion–ion correlation was shown to be the fundamental attraction, supplemented by dispersion interaction for polarizable ions such as Ca2+ and Ba2+, or metal–ligand complexes for ions such as Cu2+, Al3+ and Fe3+. Ions that form strong complexes induce local acidic environments that also contribute to the assembly. These findings are summarized in a comprehensive semi-quantitative model and are important for the design of nanomaterials and for understanding biological systems where specific ions are involved.


 Please wait while we load your content...
Please wait while we load your content...