Three consecutive steps over the chirally modified Pt surface: asymmetric catalytic cascade reaction of 2-nitrophenylpyruvates
Abstract
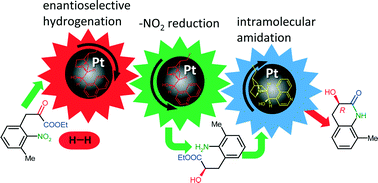
The influence of the reaction conditions on the asymmetric heterogeneous cascade reaction of 2-nitrophenylpyruvates over Pt catalysts modified with cinchonidine leading to (R)-3-hydroxy-3,4-dihydroquinolin-2(1H)-one derivatives has been studied. Results of studies on the amount of acetic acid or catalyst, nature of the Pt support, kinetic examinations, effect of H2 pressure, and modifier and substrate concentrations showed that all three steps of this catalytic cascade take place on the Pt surface, with the nitro group reduction immediately following the enantioselective hydrogenation of the keto group, whereas the final intramolecular amidation was preceded by desorption after complete reduction of the substrate and re-adsorption of the corresponding intermediate.
- This article is part of the themed collection: Catalysis on Chiral Surfaces: From Fundamental Aspects to Application

 Please wait while we load your content...
Please wait while we load your content...