Adsorption and stability of chiral modifiers based on 1-(1-naphthyl)-ethylamine for Pt catalysed heterogeneous asymmetric hydrogenations†
Abstract
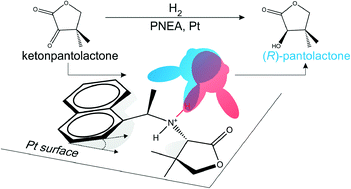
Synthetic chiral modifiers suitable for modular build-up are highly desirable for tuning the efficiency and extending the versatility of asymmetric hydrogenations on chirally-modified metal catalysts. Adsorptive anchoring and structural stability of the simple chiral modifier (R)-1-(1-naphthyl)-ethylamine [(R)-NEA] and the upgraded, secondary amine chiral modifier (R,S)-pantoylnaphthylethylamine [(R,S)-PNEA] have been investigated under catalytic hydrogenation conditions. Using attenuated total reflection-infrared (ATR-IR) spectroscopy the adsorption modes of (R)-NEA and (R,S)-PNEA at the solid–liquid interface of a technical 5 wt% Pt/Al2O3 catalyst were investigated. In addition to the naphthalene group, (R,S)-PNEA is also anchored to Pt through its pantoyl moiety providing both enhanced anchoring and also a better defined chiral surface site for the asymmetric hydrogenation of ketopantolactone (KPL). Factors influencing the stability of NEA-based chiral modifiers are discussed. The recently discovered chiral fragmentation product of (R,S)-PNEA, (S)-amino-4,4-dimethyl-dihydrofuran-2-one [(S)-AF] is shown to play no role in conferring enantioselectivity in the asymmetric hydrogenation of KPL.
- This article is part of the themed collection: Catalysis on Chiral Surfaces: From Fundamental Aspects to Application

 Please wait while we load your content...
Please wait while we load your content...