Efficient synthesis of supported proline catalysts for asymmetric aldol reactions†
Abstract
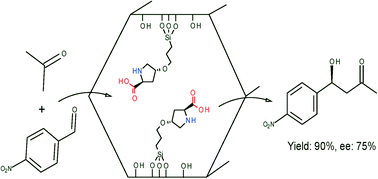
Proline has been grafted onto silica supports in a single step by reacting trans-4-hydroxy-L-proline with chloropropyl tethers, without the use of protecting groups for the proline amine and carboxylic acid functional groups. The resulting catalysts have been characterised to show that grafting is through reaction with the 4-hydroxy group. The catalysts have been tested in an asymmetric aldol reaction, and shown to be both more active and more enantioselective than equivalent catalysts prepared using a protection/deprotection route for the proline grafting step.
- This article is part of the themed collection: Catalysis on Chiral Surfaces: From Fundamental Aspects to Application

 Please wait while we load your content...
Please wait while we load your content...