Enantioselective synthesis of 1,2,4-triazolines catalyzed by a cinchona alkaloid-derived organocatalyst†
Abstract
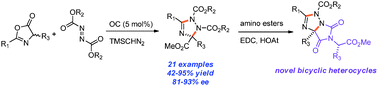
An enantioselective organocatalytic process for the one-step synthesis of poly-substituted 1,2,4-triazolines is reported. The heterocycle formation is believed to go through a step-wise mechanism of nucleophilic addition of an azlactone to an azodicarboxylate in the presence of an organic base catalyst, followed by a TMSCHN2 mediated heterocyclization. Both theoretical calculations and experimental evidence suggest the pre-organization of the transition state for the chirality determining step via a unique 7-membered intramolecular hydrogen bonding.

 Please wait while we load your content...
Please wait while we load your content...