Synthesis of novel 3-hydroxy-2-naphthoic hydrazones as selective chemosensors for cyanide ions†
Abstract
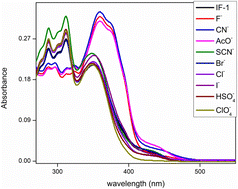
The development of an effective and selective chemosensor for CN− ions has become the need of the hour due to their hazardous impact on the environment and humans. Herein, we report the synthesis of two novel chemosensors, IF-1 and IF-2 based on 3-hydroxy-2-naphthohydrazide and aldehyde derivatives that have shown selective sensing of CN− ions. IF-2 exhibited exclusive binding with CN− ions that is further confirmed by the binding constant value of 4.77 × 104 M−1 with a low detection limit (8.2 μM). The chemosensory potential is attributed to deprotonation of the labile Schiff base center by CN− ions that results in a color change from colorless to yellow as visible by the naked eye. Accompanying this, a DFT study was also performed in order to find the interaction between the sensor (IF-1) and its ions (F−). A notable charge transfer from 3-hydroxy-2-naphthamide to 2,4-di-tert-butyl-6-methylphenol, was indicated by the FMO analysis. The QTAIM analysis revealed that in the complex compound, the strongest pure hydrogen–hydrogen bonding was observed between H53 and H58, indicated by a ρ value of +0.017807. Due to its selective response, IF-2 can be successfully used for making test strips for the detection of CN− ions.


 Please wait while we load your content...
Please wait while we load your content...