Ruthenaelectro-catalyzed C–H acyloxylation for late-stage tyrosine and oligopeptide diversification†
Abstract
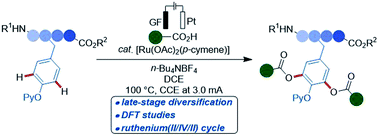
Ruthenaelectro(II/IV)-catalyzed intermolecular C–H acyloxylations of phenols have been developed by guidance of experimental, CV and computational insights. The use of electricity bypassed the need for stoichiometric chemical oxidants. The sustainable electrocatalysis strategy was characterized by ample scope, and its unique robustness enabled the late-stage C–H diversification of tyrosine-derived peptides.


 Please wait while we load your content...
Please wait while we load your content...