(Fluoro)alkylation of alkenes promoted by photolysis of alkylzirconocenes†
Abstract
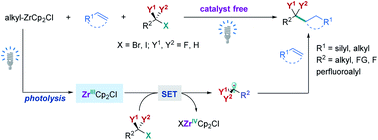
Difluoroalkylated compounds have important applications in pharmaceutical, agrochemical, and materials science. However, efficient methods to construct the alkylCF2–alkyl bond are very limited, and the site-selective introduction of a difluoromethylene (CF2) group into an aliphatic chain at the desired position remains challenging. Here, we report an unprecedented example of alkylzirconocene promoted difluoroalkylation of alkyl- and silyl-alkenes with a variety of unactivated difluoroalkyl iodides and bromides under the irradiation of visible light without a catalyst. The resulting difluoroalkylated compounds can serve as versatile synthons in organic synthesis. The reaction can also be applied to activated difluoroalkyl, trifluoromethyl, perfluoroalkyl, monofluoroalkyl, and nonfluorinated alkyl halides, providing a general method to controllably access fluorinated compounds. Preliminary mechanistic studies reveal that a single electron transfer (SET) pathway induced by a Zr(III) species is involved in the reaction, in which the Zr(III) species is generated by the photolysis of alkylzirconocene with blue light.


 Please wait while we load your content...
Please wait while we load your content...