Concise total syntheses of two flavans and structure revision assisted by quantum NMR calculations†
Abstract
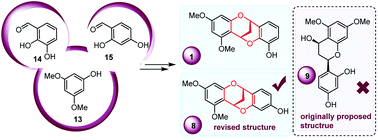
A two-step protecting-group-free protocol for the synthesis of 3′-hydroxy-5,7-dimethoxy-4-O-2′-cycloflavan (1) and concise total synthesis of 4′-hydroxy-5,7-dimethoxy-4-O-2′-cycloflavan (8) enabled by a PTSA triggered bioinspired olefin isomerization/hemiacetalization/dehydration/[3 + 3]-type cycloaddition cascade reaction are reported. The successful synthesis of cycloflavan 8 along with GIAO 13C NMR calculations of flavan-4-ol 9 and cycloflavan 8 indicated the misassignment of the flavonoid isolated previously and realized the revision of its actual structure.


 Please wait while we load your content...
Please wait while we load your content...