FeCl3-mediated selenylative benzannulation of aryl alkynones to 3-selenyl β-naphthols†
Abstract
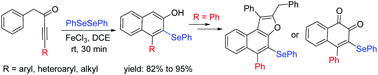
An efficient selenylative cyclization of aryl-alkynones with diselenides in the presence of iron(III)chloride at room temperature to prepare 3-seleno-2-naphthols in good yields has been described. Furthermore, the resulting products were transformed into selenyl-naphthofuran and selenyl-1,2-naphthoquinone derivatives.


 Please wait while we load your content...
Please wait while we load your content...