Synthesis of 4-(organoselenyl) oxazolones via cyclization of N-alkynyl ethylcarbamates promoted by organoselenium†
Abstract
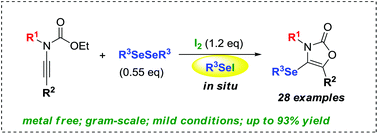
Organoselenyl iodide promoted the intramolecular nucleophilic cyclization of N-alkynyl ethylcarbamates in the synthesis of 4-(organoselenyl) oxazolones. The reaction was regioselective, giving the five-membered oxazolone products as the unique regioisomer via an initial activation of the carbon–carbon triple bond through a seleniranium intermediate, followed by an intramolecular 5-endo-dig cyclization mode. The generality of the methodology has been proven by applying the optimized reaction conditions to different organoselenyl iodides and N-alkynyl ethylcarbamates having different substituents directly bonded to the nitrogen atom and in the terminal position of the alkyne.


 Please wait while we load your content...
Please wait while we load your content...