Cheminformatic analysis of natural product-based drugs and chemical probes†
Abstract
Covering: 1981 to 2019
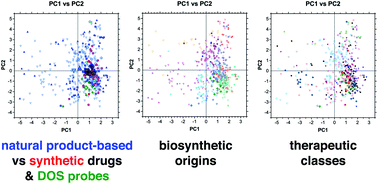
Natural products continue to play a major role in drug discovery, with half of new chemical entities based structurally on a natural product. Herein, we report a cheminformatic analysis of the structural and physicochemical properties of natural product-based drugs in comparison to top-selling brand-name synthetic drugs, and a selection of chemical probes recently discovered from diversity-oriented synthesis libraries. In this analysis, natural product-based drugs covered a broad range of chemical space based on size, polarity, and three-dimensional structure. Natural product-based structures were also more prevalent in top-selling drugs of 2018 compared to 2006. Further, the drugs clustered well according to biosynthetic origins, but less so based on therapeutic classes. Macrocycles occupied distinctive and relatively underpopulated regions of chemical space, while chemical probes largely overlapped with synthetic drugs. This analysis highlights the continued opportunities to leverage natural products and their pharmacophores in modern drug discovery.
- This article is part of the themed collection: Synthetic strategies for mining the information-rich content of natural products


 Please wait while we load your content...
Please wait while we load your content...