Convenient construction of polycyclic architectures via multicomponent reaction of amino acids, dialkyl but-2-ynedioates and 2-(o-hydroxyarylidene)-1,3-indanediones†
Abstract
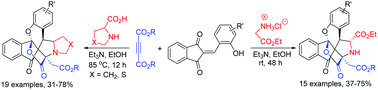
Triethylamine promoted one-pot three-component reaction of alkyl glycinate hydrochloride, dialkyl but-2-ynedioate and 2-(2-hydroxybenzylidene)-1,3-indanediones in ethanol at room temperature efficiently resulted in unique 8a 14-(epoxymethano)indeno[1′,2′:2,3]chromeno[4,3-a]pyrrolizines in satisfactory yields. On the other hand, the base promoted four-component reaction of dialkyl but-2-ynedioate, salicylaldehydes, 1,3-indanediones and secondary α-amino acids such as proline, thiazolidine-4-carboxylic acid and sarcosine in refluxing ethanol gave complex N,O,S-containing polyheterocyclic compounds in good yields. The reaction mechanism included a cascade reaction process of the in situ generation of a special kind of azomethine ylide, 1,3-dipolar cycloaddition and annulation of phenoxide.


 Please wait while we load your content...
Please wait while we load your content...