Pt–Cu nanoalloy catalysts: compositional dependence and selectivity for direct electrochemical oxidation of formic acid†
Abstract
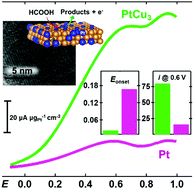
Tuning catalytic activity without increasing the platinum (Pt) load in electrode catalysts is one of the essential steps to realizing a viable fuel cell technology on a larger scale. The formation of a Pt-alloy phase with various compositions may enhance the catalytic activity without increasing the Pt load. In this work, we report the compositional dependence of Pt–Cu nanoalloy catalysts for direct formic acid (FA) oxidation without changing the Pt load. Pt–Cu nanoalloy catalysts with various compositions (Pt : Cu = 3 : 1, 1 : 1 and 1 : 3) were prepared by a thermal reduction method using stoichiometric amounts of Pt and Cu precursors at various set temperatures (500 and 800 °C), where the Pt loading (5 wt%) was maintained the same for all the tested catalytic compositions. The formation of the Pt–Cu alloy phase was confirmed by pXRD as the characteristic diffraction peaks were shifted to higher diffraction angles when compared with those of the pure phase of Pt. The average particle sizes of Pt3Cu, PtCu and PtCu3 are 5, 3, and 20 nm, respectively. Although of larger particle size and lower electrochemical surface area than commercial Pt and the other Pt3Cu and PtCu catalysts, the PtCu3 nanoalloy catalyst showed a much-improved direct FA oxidation performance both in terms of mass and specific catalytic activity when compared with the other catalysts, implying the intrinsic catalytic activity of the PtCu3 nanoalloy phase. The Pt–Cu nanoalloy catalysts showed selectivity for the direct electrochemical oxidation of FA over other fuels (e.g., methanol and ethanol). Although the multi-cycle performance of all the Pt–Cu nanoalloy catalysts decreased with the increase of the number of cycles, the catalytic performance of the PtCu3 nanoalloy catalyst was still higher in the tested 1000 cycles when compared with that of the other Pt3Cu and PtCu nanoalloy catalysts and the commercial Pt catalyst.


 Please wait while we load your content...
Please wait while we load your content...