N-type thermoelectric Ag8SnSe6 with extremely low lattice thermal conductivity by replacing Ag with Cu†
Abstract
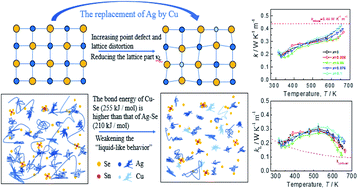
Argyrodite family compounds inherently possess low lattice thermal conductivity (κL) due to the liquid-like behavior of cations and the intimate interplay among mobile ions. Hence, they have become the focus of discussion in thermoelectrics recently. However, the major bottleneck for further improvement of their thermoelectric (TE) performance is their low carrier concentration. In this work, we take an advantage of the unique structure of Ag8SnSe6, in an attempt to further reduce the lattice part (κL) while at the same time improve their electrical property. The results show that the κL value reduces from 0.17 W K−1 m−1 to 0.12 W K−1 m−1 when Ag is substituted for Cu through induced point defects and lattice distortion and that the power factor (PF) increases from 4.1 μW cm−1 K−2 to 4.4 μW cm−1 K−2 at 645 K after enhancing the Seebeck coefficients. Finally, the maximum ZT value of ∼0.85 is attainted for Ag7.95Cu0.05SnSe6 at 645 K, an increase by a factor of 1.3 compared to that of the pristine Ag8SnSe6. This result demonstrates that the replacement of Ag by Cu in Ag8SnSe6 is an effective way to improve its TE performance.


 Please wait while we load your content...
Please wait while we load your content...