Porous BMTTPA–CS–GO nanocomposite for the efficient removal of heavy metal ions from aqueous solutions†
Abstract
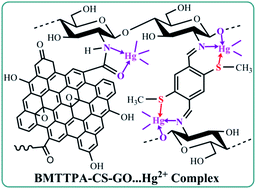
In this study, a stable, cost-effective and environmentally friendly porous 2,5-bis(methylthio)terephthalaldehyde–chitosan–grafted graphene oxide (BMTTPA–CS–GO) nanocomposite was synthesized by covalently grafting BMTTPA–CS onto the surfaces of graphene oxide and used for removing heavy metal ions from polluted water. According to well-established Hg2+–thioether coordination chemistry, the newly designed covalently linked stable porous BMTTPA–CS–GO nanocomposite with thioether units on the pore walls greatly increases the adsorption capacity of Hg2+ and does not cause secondary pollution to the environment. The results of sorption experiments and inductively coupled plasma mass spectrometry measurements demonstrate that the maximum adsorption capacity of Hg2+ on BMTTPA–CS–GO at pH 7 is 306.8 mg g−1, indicating that BMTTPA–CS–GO has excellent adsorption performance for Hg2+. The experimental results show that this stable, environmentally friendly, cost-effective and excellent adsorption performance of BMTTPA–CS–GO makes it a potential nanocomposite for removing Hg2+ and other heavy metal ions from polluted water, and even drinking water. This study suggests that covalently linked crucial groups on the surface of carbon-based materials are essential for improving the adsorption capacity of adsorbents for heavy metal ions.


 Please wait while we load your content...
Please wait while we load your content...