Asymmetric formal synthesis of (−)-tetrazomine†
Abstract
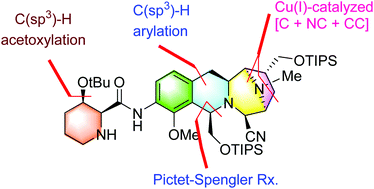
The asymmetric formal synthesis of (−)-tetrazomine is described. Key features of the synthesis include the efficient synthesis of (2S,3R)-3-hydroxypipecolic acid via a Pd(II)-catalyzed methylene C(sp3)–H acetoxylation, the stereoselective construction of the tetrahydroisoquinoline skeleton via Pd(II)-catalyzed C(sp3)–H monoarylation and Pictet–Spengler reactions, and a Cu(I)-catalyzed exo-selective asymmetric [C + NC + CC] coupling reaction to build the chiral pyrrolidine scaffold.


 Please wait while we load your content...
Please wait while we load your content...