Trichloranoids A–D, antimalarial sesquiterpenoid trimers from Chloranthus spicatus†
Abstract
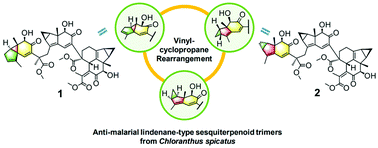
Four new lindenane sesquiterpenoid trimers, designated as trichloranoids A–D (1–4), along with a known analogue (5) were isolated from Chloranthus spicatus. Their structures were elucidated by a combination of diverse methods, including DP4+ probability and TDDFT-ECD calculations. Compounds 1 and 2 incorporated one unprecedented skeletal unit respectively in their western hemisphere, and each of them was likely formed biogenetically via a biradical rearrangement of the vinylcyclopropane. Compounds 1, 4, and 5 exhibited moderate antimalarial activities.


 Please wait while we load your content...
Please wait while we load your content...