Gold(i)-catalyzed redox transformation of o-nitroalkynes with indoles for the synthesis of 2,3′-biindole derivatives†
Abstract
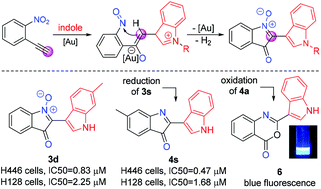
An efficient and practical method for the synthesis of 2-indolyl indolone N-oxides via gold(I)-catalyzed cascade reaction of o-nitroalkynes with indoles has been reported. The generated product could be readily converted into the 2-indolylbenzoxazinone, which emits strong blue fluorescence. We also investigated the antitumor activity of these synthesized compounds in small cell lung cancer (SCLC), and the results show that compounds 3d and 4s exhibit high anticancer potency against SCLC cells.


 Please wait while we load your content...
Please wait while we load your content...