Lewis acid mediated cyclization: synthesis of 2 spirocyclohexylindolines†
Abstract
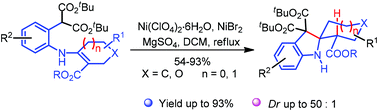
Herein, we report the synthesis of 2-spirocyclohexylindolines based on a Lewis acid mediated cyclization. This diastereoselective procedure provides the target structures in a straightforward way via dual activation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...