An entry to 2-(cyclobut-1-en-1-yl)-1H-indoles through a cyclobutenylation/deprotection cascade†
Abstract
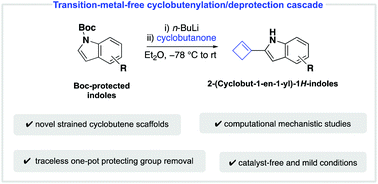
A transition-metal-free strategy for the synthesis of 2-(cyclobut-1-en-1-yl)-1H-indoles under mild conditions is described herein. A series of substituted 2-(cyclobut-1-en-1-yl)-1H-indoles are accessed by a one-pot cyclobutenylation/deprotection cascade from N-Boc protected indoles. Preliminary experimental and density functional theory calculations suggest that a Boc-group transfer is involved in the underlying mechanism.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...