Transition metal-free formal hydro/deuteromethylthiolation of unactivated alkenes†
Abstract
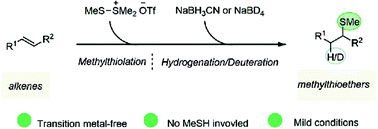
Methylthioether is involved in the methylthiotransfer process in organisms, and therefore its functionality is of paramount importance to living organisms. Several methods for the installation of the methylthio group in small molecules have been reported previously; however, procedures starting from unactivated alkenes are rare. Herein, we report a formal hydro/deuteromethylthiolation of alkenes by using dimethyl(methylthio)sulfonium trifluoromethanesulfonate as the stimulator and sodium borohydride/deuteride as the hydrogen/deuterium source. The process represents a mild, transition metal-free and methanethiol-free route towards the synthesis of methylthioethers from unactivated alkenes.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...