Rotaxanes comprising cyclic phenylenedioxydiacetamides and secondary mono- and bis-dialkylammonium ions: effect of macrocyclic ring size on pseudorotaxane formation†
Abstract
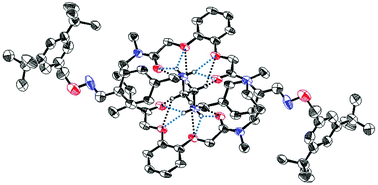
In this study we synthesized three macrocyclic tetralactams 1, possessing two phenylenedioxydiacetamide units and various spacers, for use as hydrogen bond accepting hosts for mono- and bis-dialkylammonium ions. The cyclic tetralactam 1b, featuring propylene spacers, bound secondary mono- and bis-dialkylammonium ions efficiently to form corresponding pseudo[2]rotaxanes stabilized through hydrogen bonding between the two components. The cyclic tetralactams bearing other spacers (1a and 1d) also bound these dialkylammonium ions, but the macrocycle 1a (ethylene spacer) predominantly constructed a face-to-face complex, while the macrocycle 1d (3-oxypentalene spacer) formed a non-selective complex. We synthesized three [2]rotaxanes, through imine and oxime formation, from pseudo[2]rotaxanes self-assembled from the propylene-spacer tetralactam 1b and mono- and bis-alkylammonium ions. 1H NMR spectroscopy and X-ray crystallography revealed that hydrogen bonds between the dumbbell-shaped and macrocyclic components restricted the conformation of the amide units in the [2]rotaxanes in solution and in the solid state.


 Please wait while we load your content...
Please wait while we load your content...