Stereoselective assembly of 3,4-epoxypyrrolines via nucleophilic addition induced domino cyclization of 6-halo-1-oxa-4-azahexatrienes†
Abstract
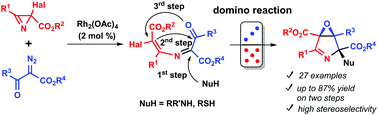
A two-step method for the preparation of 3,4-epoxypyrroline derivatives (6-oxa-3-azabicyclo[3.1.0]hex-2-enes) from 2-halo-2H-azirine-2-carboxylates, diazo keto esters, and amines has been developed. 6-Halo-1-oxa-4-azahexa-1,3,5-trienes, prepared in the first step from the azirines and diazo compounds under Rh(II) catalysis, were subjected to nucleophile-induced tandem cyclization to afford highly functionalized rac-(1R,4R,5S)-6-oxa-3-azabicyclo[3.1.0]hex-2-enes in good yields. The stereochemical outcome of the tandem cyclization induced by the secondary amines is rationalized in terms of the structural rigidity of the betaine-type precursor due to the hydrogen bonding between the ammonium group and ester group adjacent to the halogen atom. Post-modification of the 3,4-epoxypyrrolines by the Stille cross-coupling, reduction, and UV-irradiation was also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...