Domino synthesis of fully substituted pyridines by silver-catalyzed chemoselective hetero-dimerization of isocyanides†
Abstract
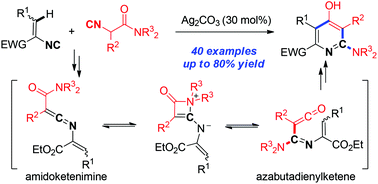
A silver-catalyzed hetero-dimerization of various vinyl isocyanides with isocyanoacetamides has been developed. This multistep domino reaction provides a facile protocol for the expedient synthesis of fully substituted pyridines in a single operation from readily available starting materials. An amidoketenimine and an azabutadienylketene are proposed as the key intermediates involved in this domino transformation. In addition, the resulting polysubstituted pyridines can be conveniently converted to pyridine-fused polycyclic frameworks.


 Please wait while we load your content...
Please wait while we load your content...