Excited-state intramolecular proton transfer in the kinetic-control regime†
Abstract
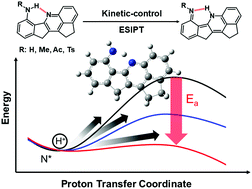
A new series of molecules bearing a 2,11-dihydro-1H-cyclopenta[de]indeno[1,2-b]quinoline (CPIQ) chromophore with the N–H⋯N type of intramolecular hydrogen bond are strategically designed and synthesized, among which CPIQ-OH, CPIQ-NHAc and CPIQ-NHTs in solution exhibit a single emission band with an anomalously large Stokes shift, whereas CPIQ-NH2 and CPIQ-NHMe show apparent dual-emission property. This, in combination with time-resolved spectroscopy and the computational approach, leads us to conclude that CPIQ-OH, CPIQ-NHAc and CPIQ-NHTs undergo ultrafast, highly exergonic excited-state intramolecular proton transfer (ESIPT), while a finite rate of ESIPT is observed for CPIQ-NH2 and CPIQ-NHMe with a time constant of 117 ps and 39 ps, respectively, in acetonitrile at room-temperature. Further temperature-dependent studies deduce an appreciable ESIPT barrier for CPIQ-NH2 and CPIQ-NHMe. Different from most of the barrier associated ESIPT molecules that are commonly in the thermodynamic-control regime, i.e. found in the thermal pre-equilibrium between excited normal and proton-transfer tautomer states, CPIQ-NH2 and CPIQ-NHMe cases are in the kinetic-control regime where ESIPT is irreversible with a significant barrier. The barrier is able to be tuned by the electronic properties of the –R group in the NR–H proton donor site, resulting in ratiometric fluorescence for normal versus tautomer emission.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...