Anisotropy of Pt nanoparticles on carbon- and oxide-support and their structural response to electrochemical oxidation probed by in situ techniques†
Abstract
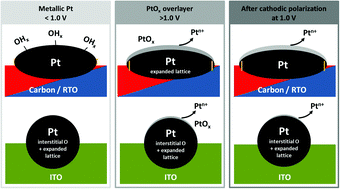
Identifying the structural response of nanoparticle–support ensembles to the reaction conditions is essential to determine their structure in the catalytically active state as well as to unravel the possible degradation pathways. In this work, we investigate the (electronic) structure of carbon- and oxide-supported Pt nanoparticles during electrochemical oxidation by in situ X-ray diffraction, absorption spectroscopy as well as the Pt dissolution rate by in situ mass spectrometry. We prepared ellipsoidal Pt nanoparticles by impregnation of the carbon and titanium-based oxide support as well as spherical Pt nanoparticles on an indium-based oxide support by a surfactant-assisted synthesis route. During electrochemical oxidation, we show that the oxide-supported Pt nanoparticles resist (bulk) oxide formation and Pt dissolution. The lattice of smaller Pt nanoparticles exhibits a size-induced lattice contraction in the as-prepared state with respect to bulk Pt but it expands reversibly during electrochemical oxidation. This expansion is suppressed for the Pt nanoparticles with a bulk-like relaxed lattice. We could correlate the formation of d-band vacancies in the metallic Pt with Pt lattice expansion. PtOx formation is strongest for platelet-like nanoparticles and we explain this with a higher fraction of exposed Pt(100) facets. Of all investigated nanoparticle–support ensembles, the structural response of RuO2/TiO2-supported Pt nanoparticles is the most promising with respect to their morphological and structural integrity under electrochemical reaction conditions.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...