New theoretical insights into the reaction kinetics of toluene and hydroxyl radicals†
Abstract
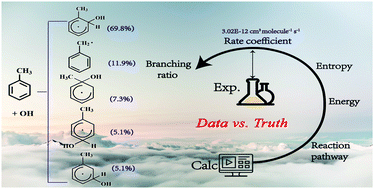
Toluene's removal mechanism in the atmosphere is mainly attributed to the OH radical, which includes major OH-addition and minor H-abstraction reactions. The cresols and RO2 derived from OH-adducts reacting with O2 have significant impacts on the generation of secondary organic aerosols (SOA) and O3. However, computed branching ratios of various OH-adducts at various theoretical levels are largely inconsistent, mainly because previously reported barrier heights of the OH-addition reaction showed a strong method dependence. In the present study, we demonstrate that this reaction involves a nonnegligible anharmonic effect (during the process of movement of OH to the benzene ring), which has been overlooked by previous studies. The reaction kinetics of toluene + OH was systematically studied by a high-level quantum chemical method (CCSD(T)-F12/cc-pVQZ-F12//B2PLYP-D3/6-311++G(d,p)) combined with RRKM/master equation simulations. The particle-in-a-box approximation was used to treat the anharmonicity in this system. The final total rate coefficient is calculated to be 3.02 × 10−12 cm3 molecule−1 s−1 at 300 K and 1 atm. The main products for toluene + OH are computed as ortho-adducts (69.8%), benzyl radical + H2O (11.9%), ipso-adduct (7.3%), para-adduct (5.1%), and meta-adduct (5.1%). Our results indicate that both high level quantum chemical calculations for the crucial barrier heights and appropriate treatments for the anharmonicity determine the accuracy of the final computed total rate coefficients and branching ratios. Further analysis of the branching ratios of various reaction channels provides insight into the atmosphere-initiated oxidation of toluene.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...