Synthesis of tri-substituted allyl alcohols via a copper/iron co-catalyzed cascade perfluoroalkylation/rearrangement of aryl propynyl ethers†
Abstract
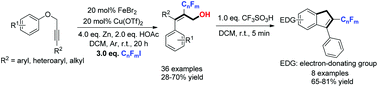
A copper/iron co-catalyzed fluoroalkylative cascade cyclization/1,4-aryl migration of aryl propynyl ethers with readily available iodoperfluoroalkane reagents for the synthesis of the corresponding perfluoroalkylated tri-substituted allyl alcohol derivatives is reported. This novel protocol provides a very mild method for the construction of perfluoroalkylated cinnamyl alcohols in one step with high regio- and stereo-selectivities. Further efficient application of this protocol is illustrated with the synthesis of 2-perfluoroalkyl-3-aryl-1H-indenes via a simple acidic workup.


 Please wait while we load your content...
Please wait while we load your content...