Caerulomycins from Actinoalloteichus cyanogriseus WH1-2216-6: isolation, identification and cytotoxicity†
Abstract
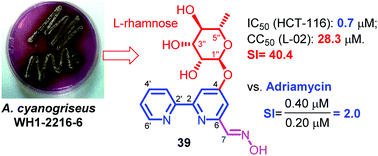
Caerulomycins are a family of natural alkaloids featuring a 2,2′-bipyridine nucleus. Since the first example of caerulomycins, caerulomycin A from Streptomyces caeruleus, was reported in 1959, there have been 34 natural caerulomycins from microorganisms reported. The unique structure of caerulomycins and their potential bioactivity, including antifungal activity, cytotoxicity, and immunosuppression, have attracted the interest of both chemists and biologists. Herein, we report 8 new caerulomycins (19–22 and 39–42) as well as 14 known analogues (1–3, 6–9, 16, 18, 24, 25, 27, 29, and 31) from wild-type and mutant Actinoalloteichus cyanogriseus WH1-2216-6. The structures including the absolute configurations were unambiguously elucidated by spectroscopic analysis, X-ray single crystal diffraction, 1-phenyl-3-methyl-5-pyrazolone (PMP)-labeling HPLC analysis, Mosher's method and quantum electronic circular dichroism (ECD) calculation. The cytotoxicity of caerulomycins 1–42 and some previously reported analogues was assayed against six human tumor cell lines and a human normal liver cell line, L-02. Compounds 1, 2, 5, 13, 18, 27, and 39 exhibited moderate to strong cytotoxicity against the tumor cells with IC50 values ranging from 0.1 to 50 μM. The structure and activity relationships (SARs) are also discussed. In addition, the caerulomycin glycosides (29, 34, and 39) showed effective selectivity against HCT-116 cells with low toxicity toward the normal L-02 cell line, indicating their potential use as lead compounds for antitumor drugs.


 Please wait while we load your content...
Please wait while we load your content...