Rapid construction of tetrahydropyridine scaffolds via formal imino Diels–Alder reactions of Schiff bases and Nazarov reagents†
Abstract
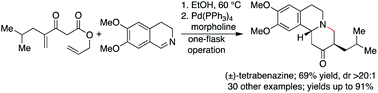
Described is a one-flask, two-step method for the synthesis of highly functionalized piperidines. The process involves formal [4 + 2] cycloadditions of Schiff bases and Nazarov reagents, followed by facile elaborations of the initial cycloadducts. Notably, these aza-annulations are facilitated by protic solvents and proceed smoothly under ambient conditions, without other additives. The synthetic utility of this annulation protocol is further showcased through a concise, convergent synthesis of (±)-tetrabenazine.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...