Synthesis of new cyclazines and 4,5-diaryl-1H-pyrrol-3(2H)-one units in discoipyrroles from indolizinone-DMAD cycloadducts†
Abstract
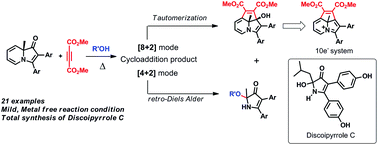
The reaction of indolizinones with dimethyl acetylenedicarboxylate gave direct access to 3′,8a-dihydrocyclopenta[hi]indolizin-8a-ol and 1H-pyrrol-3(2H)-one in good yields. The former skeleton is a precursor to cyclazines with nitrogen on the periphery, a hitherto un-accessed 10-π system. Their formation involves initial [4 + 2] or [8 + 2] modes of cycloadditions; the retro-Diels Alder reaction of the [4 + 2] cycloadduct leads to 1H-pyrrol-3(2H)-one, whereas [8 + 2] addition followed by π-reorganization leads to the azatricyle. Analysis of substituent effects on product distribution showed that electron donating groups on the C3-aryl ring promote the formation of the azatricycle preponderantly. Treatment of one of these azatricycles (3c) with HBF4 led to the formation of the corresponding 10e-aromatic species which was detected by NMR spectroscopy. In addition, formation of the 1H-pyrrol-3(2H)-one skeleton through the normal retro-Diels Alder pathway was employed in the total synthesis of Discoipyrrole C, which is a new lead against lung cancer.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...