Bypassing the stereoselectivity issue: transformations of Kinugasa adducts from chiral alkynes and non-chiral acyclic nitrones†
Abstract
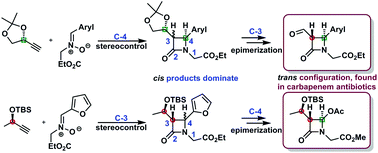
An approach to β-lactams via a reaction between chiral copper acetylides and acyclic nitrones is reported. Electronic circular dichroism (ECD) in combination with NMR spectroscopy was used to determine the absolute configuration of all components of complex mixtures of azetidinones. Stereochemical preferences observed in the studied reactions are discussed and a model of a new reaction pathway supported by DFT conformational analysis is proposed. Subsequent transformations of the synthesized Kinugasa adducts followed by epimerization at the C-3 carbon atom led to trans-substituted azetidinones with improved stereoselectivity, mimicking a variety of important β-lactam structures. On the other hand, the oxidation of the furyl residue to the carboxylic group followed by oxidative decarboxylation with lead tetraacetate afforded the more thermodynamically stable trans-substituted 4-acetoxy azetidinone. The latter strategy is particularly attractive for the diastereomeric mixture in which isomers with the same configuration at the C-3 carbon atom dominate.


 Please wait while we load your content...
Please wait while we load your content...